UofT Solar Fuels Cluster
The U of T Solar Fuels Cluster is an interdisciplinary research team devoted to developing scalable, cost effective materials solutions towards using CO2 as a chemical feedstock for valuable products. Leveraging the expertise of some of Canada's leading chemists, engineers, and material scientists, we hope to initiate a paradigm-shifting zero-emission CO2 economy.
August 23rd 2022: Congratulations to Professor Ozin on his birthday paper on CO2 photocatalysis in Matter!
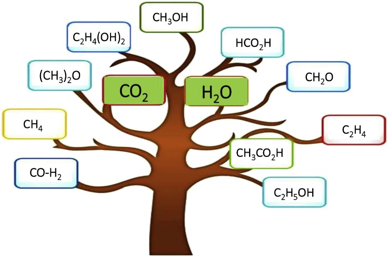
Today is Geoff’s 79th birthday. Besides celebrating it online with family, friends, colleagues and alumni on the topic of “ride the (Direct Air Capture) train, save the world”, a birthday perspective paper entitled “Accelerated optochemical engineering solutions to CO2 photocatalysis for a sustainable future” is online today.
In this perspective, Geoff systematically looked at the past and present of gas-phase heterogeneous CO2 photocatalysis by introduction of the background and summary on the state-of-the-arts.
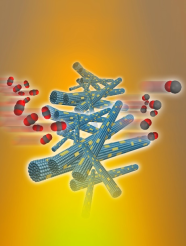
Geoff points out that the vision of CO2 photocatalysis is to produce a pyramid of fundamental chemicals from CO2 and H2O to counter the climate change as illustrated in above picture, and currently, there are four main directions in seeking for high solar-to-chemical energy efficiency: 1) Materials engineering strategies and new reactor designs for enhanced-performance heterogeneous CO2 photocatalysis; 2) Computational modeling of design strategies for studying light and heat transport in photocatalytic CO2 hydrogenation processes; 3) Comparison of thermochemical, photochemical, and photothermal CO2 utilization strategies and future opportunities; 4) Determining key technoeconomic decision-making criteria for assessing photocatalytic process viability and potential the cost of a mole of photons to generate a mole of product is a pivotal metric to decide whether or not the industrialization of CO2 photocatalysis is technologically and economically viable.
Based on this, Geoff proposes that the accelerated development of the CO2 photocatalysis is necessary given the looming climate change challenge. The vision is based on revolutionary scientific advances of self-driving laboratories that are expected to creatively integrate artificial intelligence, machine learning, robotic automation, and data management tools to accelerate the discovery of high efficiency photocatalysts and photoreactors through fast, low-cost, and parallel rather than slow and expensive serial procedures. See full story at Matter .
July 18th, 2022: Sand batteries that are dirt cheap

Have you ever wondered how much solar heat is stored in the deserts of the world and wasted. Imagine if it could be utilized ! We all know under the threat of climate change that cost-effective energy storage solutions is a world-wide objective to enable the transition from fossil to renewable forms of energy. Different storage media have been discovered so far such as gravity, lithium-ion batteries, supercapacitors, and molten salts. Recently, a Finnish start-up company Polar Night Energy demonstrated the potential of ultra-cheap sand for energy storage, which is totally green with reported power up to 100 MW, capacity up to 20 GWh, and efficiency as high as 99 %. While it sounds like too good to be true, the core idea is to use solar or wind electricity to heat sand to 600-1000 ℃ and reuse the thermal energy to heat buildings or regenerate electricity. Then here goes one of the most fascinating points—the thermal energy can be stored for months before it degrades. Furthermore, the infrastructure of the sand system is straightforward, sands and pipes in cylinders at smaller scales or sands and pipes in canyons at larger scales, with vacuum containment for insulation. Due to abovementioned merits, the Polar Night Energy already is working to build energy storage facilities world-wide. See full story at Advanced Science News.
May 31st, 2022: Congratulations to Wei and co-authors on their recent publication on silica in the Chem Catalysis!
Besides acting as the most widely used semiconductor materials, silicon in oxide form (silica SiO2) has attracted broad interests in environmental and energy catalysis recently due to the low cost, non-toxicity and ubiquitous presence in the Earth’s crust. In this Review of silica mediated catalysis, Wei and co-authors begin by brief discussions of the general applications of silica, methods to engineer it, and the mechanistic understanding during catalysis processes. This is followed by a discussion of some recent examples that silica counterintuitively goes beyond the general understandings, including interplay with optics/thermal conduction to enhance photocatalysis and geometric confinement of supported metals to show strong metal-silica support interaction. Throughout the Review the authors attempt to highlight the novel and emerging designs that broaden the limits of silica. See full story at Chem Catalysis.
April 7th, 2022: Congratulations to Wei and Geoff on their recent publication in Advanced Science and the winner of front cover!

Catalyst stability is the most important parameter for industrial application. For example, Cu is a prestigious CO2 catalyst, but its durability is partially limited by the sintering of Cu nanoparticles under high temperature. Increasing the Cu-support interaction can enhance stability, which is typically realized on reducible metal oxides via in-situ migration to partial Cu coverage. In a recent work, Wei and Geoff developed an alternative method to expand the strong metal-support interaction to non-reducible silica supported Cu. Within the novel design, a two-dimensional multi-layer SiO2 support is prepared by topological exfoliation of CaSi2 with CuCl2 and thereafter calcination Cu nanoparticles, and thus Cu nanoparticles are encapsulated and confined between layers. The prepared Cu-SiO2 catalysts exhibit excellent activity and long-term stability in high-temperature CO2 hydrogenation reactions. See full story at Advanced Science.
April 7th, 2022: Decarbonizing the chemical industry with sustainable photons

Chemical industry is a stubborn sector for decarbonization but the benefits could be enormous. To cut the emission of chemical industry, current solution is electrification of which the power is supplied by grid electricity, while the long-term goal is to develop pure renewable energy-driven carbon-neutral or carbon-negative techniques. To this regard, photocatalysis is among the most appealing pathways. In a recent study in ACS Energy Letters, the economic viability of photocatalytic chemical production is found to be promoted by reduced cost of renewable energy and enhanced efficiency of LED. See full story at Advanced Science News.
April 7th, 2022: Congratulations to Lu, Chengliang and Geoff on their recent publication in Nature Communications!
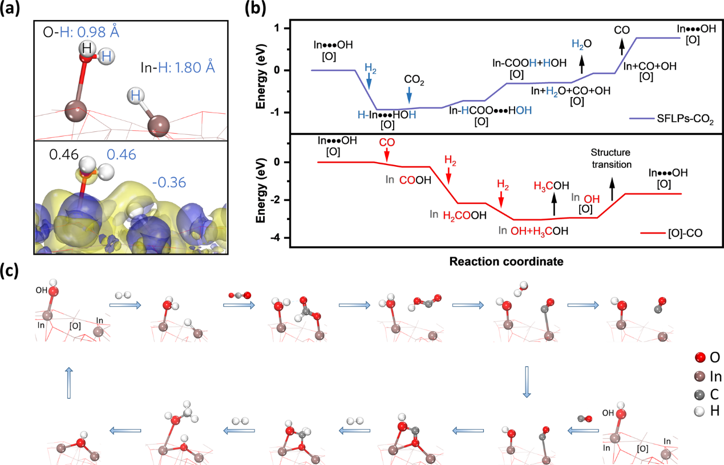
CO2-to-methanol catalysis is an appealing way for the production of solar fuels. The benchmark catalyst is the decades-old Cu/ZnO/Al2O3 of high stability and high activity, but it should be operated under high hydrogen concentration (>75 %) and high pressure to suppress the competing reverse water-gas shift side-reaction (CO2-to-CO). In a recent work, Lu, Chengliang, and Geoff showcased a black In2 O3-x(OH) Oy catalyst that can shift the competing CO and methanol production to cooperative one. To amplify, the hydridic In-H and protonic In-OH in the catalyst forms surface frustrated Lewis pair (SFLP) to enable facile CO2 activation to CO, with the SFLP degraded to oxygen vacancy. Fortunately, the produced CO can be captured by the oxygen vacancy and be transformed into methanol on site. After the desorption of methanol, the oxygen vacancy is recycled to SFLP upon H2 heterolysis. As a result, the methanol synthesis over black In2 O3-x(OH) Oy is promoted by the CO formation, surpassing the Cu/ZnO/Al2O3 catalyst under ambient pressure and 50 % H2 concentration. See full story at Nature Communications.
April 6th, 2022: Gravity energy storage elevated to new heights

A global energy transition is expected to increase the share of renewable energy from current 12% to 90% in 2050. Different from the on-demand fossil fuel, inteminent renewable energy including solar and wind calls for large-scale, cost-effective grid-scale energy storage facilities. Currently, the energy storage is dominated by gravity-based pumped hydro (90%), followed by lithium, lead and zinc batteries (5%), and then the rest. Recently, a Swiss company Energy Vault demonstrated potential to change this framework by the use of cranes to lift and lower heavy composite blocks into massive architectures, which delivers gigawatts capacity and remarkable energy efficiency of 83-85%. See full story at Advanced Science News.
March 16th, 2022: Understanding the science behind the neon shortage
The war in Ukraine has araised the global concern for possible chip shortage due to disruption in semiconductor-level neon supply. Being the world’s major neon supplier, Ukraine perfects the process of concentrating 18 ppm neon in air to 99.999span>% purity based on cryogenic fractionation. This kind of high-purity neon is used to trigger the 193 nm-deep ultraviolet wavelength for the state-of-the-art 7 nm spatial resolution photolithography, together with argon and fluorine in an excimer laser. The excited-state chemistry behind the argon-fluorine-neon excimer laser is explained in detail by Geoff, as well as why the neon is necessary during the process. See full story at . Advanced Science News.
March 16th, 2022: Congratulations to Professor Geoffrey Ozin on being selected as the 2022 recipient of the Killam Prize in natural sciences!

It is with great delight and excitement to announce that Professor Geoffrey Ozin is the 2022 Killam Prize winner for his leading work in nanoscience. The Killam Prizes are awarded by the Canada Council for the Arts and among Canada’s most prestigious prizes for careers in research, which is to “recognize and celebrate our most inspiring scholars and thought leaders.” Five awards are given, one each in the fields of humanities, social sciences, natural sciences, health sciences, and engineering. Each of the Laureates receive $100,000 in award funding.
February 19th, 2022: Congratulations to Meikun and co-authors on their recent publication in Angewandte Chemie!
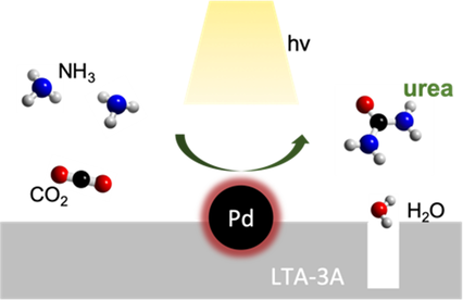
Urea is an agricultural fertilizer that nourishes humanity, and its production relies on the century-old fossil fuel-based Bosch–Meiser process. In this report, Meikun and coauthors develop the first photocatalytic urea production via a one-step reaction between two ammonia with one carbon dioxide at facile condition. The catalyst is made of supported Pd nanoparticles on molecular sieves LTA-3A. The Pd nanoparticles serve the dual function of catalyzing the dissociation of NH3 and providing the photothermal driving force for urea formation, while the absorption capacity of LTA-3A removes by-product H2O to shift the equilibrium towards urea production. The solar urea production rate is 87 µmol g-1 h-1 under ~5 suns. See full story at Angew.
February 19th, 2022: Congratulations Dr. Shufang Ji on being selected as a recipient of the 2022 Banting Postdoctoral Fellowship Award!

The Banting Postdoctoral Fellowships program is a highly competitive and prestigious award designed to provide funding to the very best postdoctoral applicants, who will positively contribute to the country's economic, social, and research-based growth. Typically, 70 fellowships per annual are awarded to more than 600 applicants from all over the world. The Solar Fuels Groups is pleased to announce that Dr. Shufang among the few selected recipients.
February 19th, 2022: Congratulations to Joel and co-authors on their recent publication in Advanced Energy Materials!
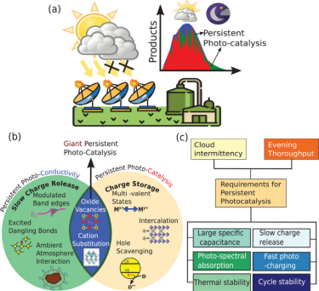
It remains a great challenge to overcome the intermittent and fluctuating sunlight for continuous photocatalytic fuels production. In this review, Joel and co-authors propose that persistent and memory-based photocatalysts, which efficiently operate under near zero light fluxes beyond sunset and during cloud intermittencies, are a potential solution to address and mitigate the challenge. Based on the idea of multivalent charge storage materials that allow charging during illumination and discharging to generate charge carriers upon post-illumination, this review describes examples of persistent photocatalysis systems, outlines the working mechanism, suggests strategies for performance optimization, proposed standardized Figures of Merit and discusses possible application scenes beyond solar fuels. See full story at Advanced Energy Materials.
February 19th, 2022: Congratulations to Lourdes, Abhinav and co-authors on their recent publication on CO2 Photocatalytic Foams in the Chemical Engineering!
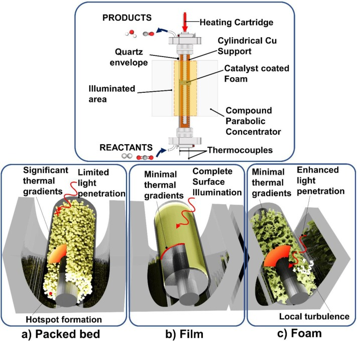
To enhance the solar-to-chemical conversion efficiency of CO2 photocatalysis, light active materials need to be engineered into multiscale architectures that maximize photon capture and minimize optical losses. In this paper, Lourdes, Abhinav and co-authors developed a photocatalytic foam that helps achieve this goal by coating a uniform distribution and thickness indium oxide hydroxide nanorods film onto the internal surface of an oxidized nickel foam. Optimizing the size and density of pores of the photocatalytic foam enabled optimization of photochemical and thermochemical contributions to the rate for the solar reverse water gas shift. See full story at Chemical Engineering Journal.
February 15th, 2022: Beheading a Century-Old Chemical Process
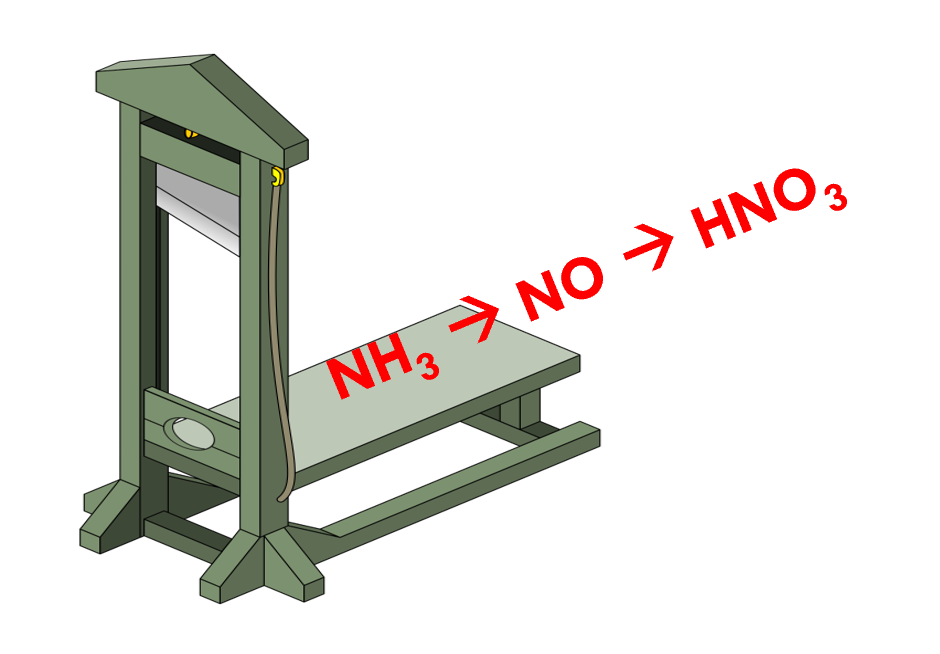
De-carbonizing the fossil-intensive chemicals and petrochemcials industries will help achieve a “net-zero” sustainable society. A recent report has targeted nitric acid production, an important feedstock for the production of dyes, pigments, nylon, explosives, and fertilizers. Currently, nitric acid is produced via catalytic ammonia synthesis and subsequent oxidation, but the high temperature and pressure (800-950℃, 12 atmospheres) and poor selectivity to NO lead to deleteriously high greenhouse gas CO2 and N2O emissions. In this work, a new two-step, chemical looping process has been invented which uses lattice oxygen instead of oxygen gas to oxidize NH3 to NO with increased selectivity (99.8%) and under milder reaction temperature (650℃). See full story at Advanced Science News .
January 18, 2022: CALF-20 - A carbon capture success story
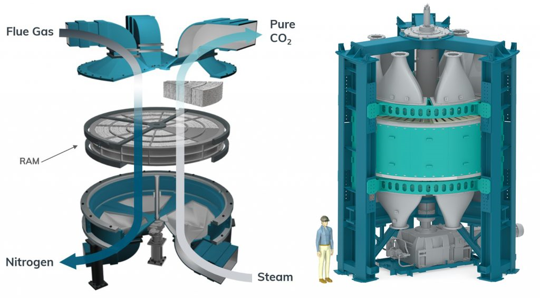
Marketable, large-scale, and energy-efficient CO2 capture is essential for a net-zero future, which would be implemented at high-emitting locations, such as power plants, metal, cement, and chemical refineries. Liquid solvents such as amines, hydroxides, and organics, and metal oxide solids including alkali and alkaline earth oxides are typical absorbents, but they suffer from energy-intensive, non-selective dilemmas. How to develop an efficient material to overcome these problems remains the key challenge. Recently, the CALF-20, an intricate layered crystal composed of zinc, triazolate, and oxalate arranged in a 3D structure filled with nanometer-scale holes, has been used by Canadian company Svante in their revolutionary carbon capture process. A gram of this material has a massive surface area of 500 square meters, and the pores weakly yet preferentially bind CO2 over water. As a result, the CALF-20 displays selective CO2 physisorption at high capacities, retains it up to and beyond 40% RH with durability over 2000 h, and only requires modest amounts of energy to remove CO2. See full story at Advanced Science News .
January 9, 2022: Congratulations to Dr. Loh and Professor Ozin on their new book Energy Materials Discovery: Enabling a Sustainable Future!

It is with great excitement and delight we announce that Energy Materials Discovery: Enabling a Sustainable Future written by Prof. Geoffrey Ozin, and Dr. Joel Loh is set to be published by Royal Society of Chemistry Publishing in May, 2022.
About the book: This book presents, through the eye of materials chemistry, an umbrella view of the myriad classes of materials that make renewable energy technologies work. They are poised to facilitate the transition from non-renewable and unsustainable energy systems of the past into renewable and sustainable energy systems of the future. It is a story that often begins in chemistry laboratories with the discovery of new energy materials. Yet, to displace materials in existing energy technologies with new ones, depends not only on the ability to design and engineer materials and technologies that exhibit superior performance metrics but also meet a demanding collection of economic, regulatory, social, policy, environmental and sustainability criteria.
January 1, 2022: Congratulations to Prof. Lu, Yuping, and Geoff on their recent publication in Journal of Energy Chemistry!
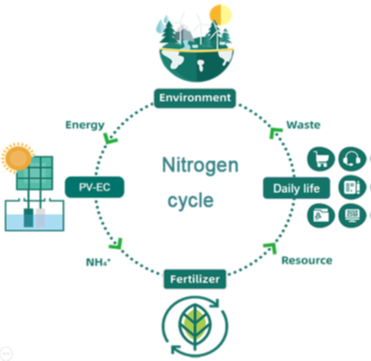
Artificial nitrogen reduction to ammonia is essential to lives on earth, offering essential nitrogen elements for biomolecules, commodity and fuel chemicals. The industrial Haber-Bosch process is established in 1913 and constitutes half of the fixed nitrogen in terms of global nitrogen cycle nowadays. However, high energy intensity, harsh reaction conditions, and extensive carbon footprint remain to be resolved. In a recent work, Prof. Lu, Yuping, and Geoff showcased an oxygen vacancy-laden tungsten oxide (WO3-x) catalyst electrochemically activating and fixing N2 molecule into ammonia in neutral electrolyte. The reaction could work with green electricity generated by photovoltage panel and the ammonia productivity could be further promoted by light irradiation. Energy and economic analyses signify the photovoltaic electrochemical WO3-x system as a feasible stand-alone green ammonia farm. See full story at Journal of Energy Chemistry.
January 1, 2022: Congratulations to Dr. Loh and Prof. Ozin on their recent publication in Nature Sustainability!
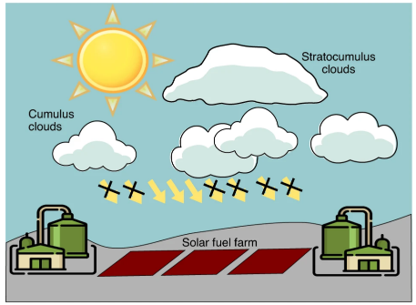
Sunlight utilization is at the central of 21st century, being of fundamental importance to energy, environmental, climate and economy securities. However, the greatest challenge is known as the “duck curve” that depicts the mismatch between peak power demand and supply in the morning, evening and overcast days. This also applies to CO2 photocatalysis where continuous, instead of intermittent, production is favorable for potential industrial use. In the recent Nature Sustainability perspective, Dr. Loh and Prof. Ozin proposed a persistent CO2 photocatalysis scenario that could overcome the intermittent dilemma. The core idea is engineering a photocatalytic and charge-storing hybrid materials system that charges during the sunlight illumination while discharges to continue the charge carries-driven catalysis upon postillumination. In the perspective, the common strategies between catalytic engineering and charge storage engineering, various combinations of material candidates, as well as their interfacing structures are discussed in detail for CO2 hydrogenation of prolonged working period. See full story at Nature Sustainability.
January 1, 2022: Will industrial-scale photocatalysis see the light of day?

90% of our commodity chemicals and fuels are made from thermocatalysis industries driven by heat or grid power. In the context of carbon-neutral society, sunlight-driven photocatalysis is an ideal alternative to thermocatalysis especially for CO2 and H2O utilization. However, how to scale the lab-scale photocatalysis into real industry remains uncertain. To amplify, the efficiency of photocatalysis not only rely on the temperature related reaction rates but also the utilization of charge carriers, which requires simultaneous optimization of quantum yield and light transport. The challenges lie in photocatalyst and photoreactor engineering to use every incident photon reaching every catalytic site while minimizing parasitic absorption, reflection, scattering, transmission, and thermal conductive, convective, and radiative losses. See full story at Advanced Science News.
December 11, 2021: Dissemination of science in a different way by “the father of nanochemistry”

Professor Geoff Ozin has disseminated scientific knowledge in and beyond academe in remarkably creative ways. In addition to writing pioneering textbooks Cryochemistry, Nanochemistry and Concepts in Nanochemistry, he has written general-audience science books, including his most recent, The Story of CO2: Big Ideas for a Small Molecule (which, in a featured interview in The Washington Post, US Energy Secretary Jennifer confirmed she was reading), with another to be published in the New Year with the Royal Society of Chemistry, 2022, Energy Materials Discovery for a Sustainable Future. He is also a constant presence in accessible science media, including the prestigious Wiley-VCH Advanced Science News – all part of his continuing leadership efforts.
December 11, 2021: Congratulations to Dr. Athan Tountas, Prof. Geoff and Sain on their recent publication in Nature Catalysis!
Solar energy and CO2 utilizations are recognized as key to counter the global warming and build a “net-zero” sustainable society, and the surging global temperature has set the deadline of establishing revolutionary techniques in several decades. The urgency requires to assess most feasible current pathways among various solar energy and CO2 proposals and stick to it progressively. Athan Tountas, Prof. Ozin and Prof. Sain recently published their perspective ‘Solar methanol energy storage’ in the Nature Catalysis focus issue ‘CO2 Reimagined’ that acknowledges the five-year anniversary of the Paris Agreement. In it they explore the feasibility of storing renewable energy in the form of intermittent solar energy as methanol and highlight the performance and efficiency advantages of using CO-rich syngas derived from commercial-ready RWGS technology compared to direct-CO2 processes. The Focus is dedicated to progressing the fundamental science and practical implementation of this technology to advance climate goals. See full story at Nature catalysis..
November 24, 2021: Congratulations to Professor Geoffrey Ozin and Dr. Mireille Ghoussoub for mention of their book, The Story of CO2, by United States Energy Secretary Jennifer Granholm in this recent Washington Post article!

November 15, 2021: Common concerns of the clean energy transition

With COP26 happening now in Glasgow, Scotland, there are still common concerns about where the money will come from to transition our economies to clean energy, the viability of carbon taxes, and how long the transition will take. By explaining the transition policy and estimating the technology development, Athan and coauthors suggest that new capacity in Canada will be funded by firms and investors and ultimately consumers, and establishing a steadily increasing carbon tax, improving solar, wind, batteries and other technologies are likely to take a few decades. See full story at Advanced Science News..
October 31, 2021: Prof. Ozin as invited speaker in international symposium on photo & electro catalytic CO2 reduction, November 8-10, 2021

In a global effort to counter the energy crisis and meet the IPCC 1.5 ℃ goal, photocatalysis and eletrocatalysis that can transform carbon dioxide into a myriad of value-added products such as fuels and feedstock chemicals, are regarded as promising solutions. In the upcoming International Symposium on Photo & electro Catalytic CO2 Reduction. held by Naikai University (China), Geoff, together with 14 world top CO2 experts are invited to discuss on the state-of-the-arts, future opportunities and challenges on the emerging field.
October 28, 2021: Scaled and directly integrated PV-EC means big news for green hydrogen

Green hydrogen is promising to decarbonize the energy, transportation and industry sectors to counter the climate crisis. But the levelized cost of any form of green hydrogen must be competitive with steam methane reforming at $2.5/kg. Recently, a cheap yet powerful water splitting device has been assembled by the Juelich Research Centre in Germany. In the device, a triple-junction thin-film a-Si:H/a-Si:H/µc-Si:H photovoltaic modules are directly coupled to an anion exchange membrane of an alkaline water electrolysis cell. The wireless device demonstrates a solar-to-hydrogen efficiency exceeding 10% at a 10 m2 scale, and less than 10% activity decrease in a 9-month test. See full story at Advanced Science News..
July 27, 2021: Nanoscale Greenhouse Enables CO2 Photocatalysis
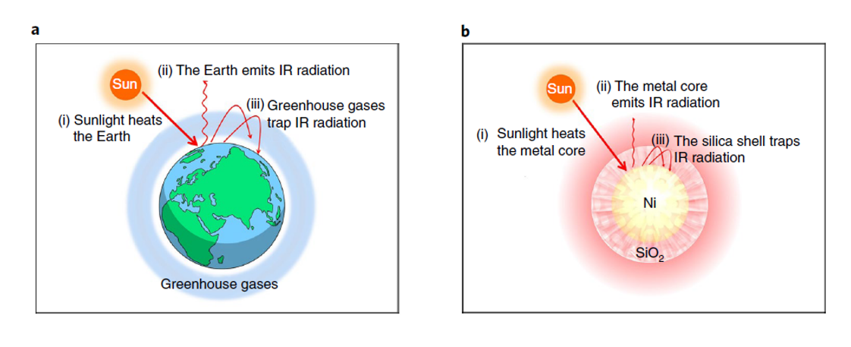
Making solar chemicals and fuels enabled by photothermal CO2 catalysis is proving to be a promising pathway to counter climate change and the energy crisis. Advanced photothermal materials design can capture the merits of broad-band spectral absorption and high efficiency in CO2 photocatalysis. In a paper published in Nature Energy, researchers from the groups of Le He, Sochow University, and Geoffrey Ozin, University of Toronto, learned how to mimic the sunlight trapping effect of a conventional greenhouse at the nanoscale, to improve the photon conversion of methanation and reverse water gas shift reactions, with spectacular results. They synthesized a hybrid nickel nanocrystal sheathed by porous silica, the heat insulation and infrared shielding effects of which confine the photothermal energy and surface chemistry to the nickel core, while CO2-H2 reactants and CO-CH4 products enter and escape through the porous shell. In essence, a nanoscale greenhouse has been discovered which facilitates high efficiency CO2 supra-photothermal catalysis. See full story at Nature Energy..
July 9, 2021: Congratulations to Jiuli and coauthors on their publication in Advanced Science!
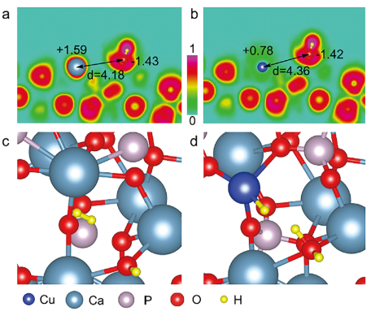
CO2 reduction to commodity chemicals and fuels holds great promise to alleviate the global warming. The realization typically requires earth-abundant catalysts and inexpensive driving force. In this paper, Jiuli and coauthors reported a Cu2+-substituted hydroxyapatite (Ca10(PO4)6(OH)2) mineral photocatalyst achieving peak CO2-to-CO performance of 215 µmol per gram catalyst per hour without CH4 byproduct. The activity originates from novel active sites of surface frustrated Lewis pairs—the proximal Lewis acidic Cu2+ and Lewis basic OH-, which selectively produce CO via a formate intermediate. See full story at Advanced Science.
July 2, 2021: Chinese Story of CO2

We are honoured and delighted to announce that The Story of CO2: Big Ideas for a Small Molecule has launched its Chinese version published by Science China Press. It is a new landmark right after the bronze medal in Indie Book Prize. The translation is carried out by Prof. Wei Sun and Prof. Le He, two experts in CO2RR and distinguished alumni of Ozin’s group.
About the book: The climate crisis requires that we drastically reduce carbon dioxide emissions across all sectors of society. The Story of CO2 contributes to this vital conversation by highlighting the cutting-edge science and emerging technologies – a number of which are already commercially available – that can transform carbon dioxide into a myriad of products such as feedstock chemicals, polymers, pharmaceuticals, and fuels. This approach allows us to reconsider CO2 as a resource, and to add "carbon capture and use" to our other tools in the fight against catastrophic climate change. See also an introduction and story by the Faculty of Science and Art, UofT, the Story of CO2 describes a groundbreaking solution to the climate crisis..
July 2, 2021: Carbon dioxide locked in stone

Under the background of carbon capture, storage, and utilization, integrating CO2 into mineral lattice is a potential pathway but how to implement it matters. Both cost efficiency and capable materials are crucial to a potential use in large scale. To this regard, magnesium and calcium oxides find their roles in carbon sequestration by mineralization to carbonates followed by materials regeneration, with overall cost as low as 156 US dollars per tonne of carbon dioxide. The cyclical process could be compatible to steel industry or direct air capture, with end uses to cement and high-purity CO2 production. See full story at Advanced Science News..
July 2, 2021: Nitrous oxide – Not a laughing matter

Nitrous oxide (N2O) is a colorless gas that can make people laugh or feel pain relief, as well as a long-term agent to warm the planet and deplete the ozone layer. Specifically, its warming impact accounts for 300 times more potent than CO2. Life cycle analysis indicates that most of the atmospheric nitrous oxide (75%) originates from agricultural soil management due to excess usage of nitrogen fertilizer. To confront the N2O crisis via a cost-effectively way, Prof. Ozin proposes a photocatalysis pathway involving HNO3 + 2 H2O + hv → NH4OH + 2 O2 in the first place. See full story at Advanced Science News..
June 20, 2021: Bronze in the INDIES book award! Congratulations to Professor Geoffrey Ozin and Dr. Mireille Ghoussoub for their book, The Story of CO2

We are delighted to announce that The Story of CO2: Big Ideas for a Small Molecule published by University of Toronto Press in Nov., 2020 has won the bronze medal in the Foreword INDIES awards in the category of Ecology and Environment! See foreword reviews for details..
About the book: The climate crisis requires that we drastically reduce carbon dioxide emissions across all sectors of society. The Story of CO2 contributes to this vital conversation by highlighting the cutting-edge science and emerging technologies – a number of which are already commercially available – that can transform carbon dioxide into a myriad of products such as feedstock chemicals, polymers, pharmaceuticals, and fuels. This approach allows us to reconsider CO2 as a resource, and to add "carbon capture and use" to our other tools in the fight against catastrophic climate change. See also an introduction and story by the Faculty of Science and Art, UofT, the Story of CO2 describes a groundbreaking solution to the climate crisis..
June 10, 2021: What do carbon capture and beer bubbles have in common?

A glass of cold beer with foamy head and sweet fizz guarantees a pleasant experience in the summer. As you may have realized that the major gas component in the bubble is the same one warming our planet, it would be a good question to ask how many CO2 bubbles are released during our toast and how much beer bubbles contribute to the global CO2 footprint. Answers to these question requires an understanding of thermodynamics and kinetics of bubble nucleation and growth, provided by a recent report, and a grain-to-glass life-cycle-analysis . See full story at Advanced Science News..
May 17, 2021: Playing ball with the Haber–Bosch process
There is a worldwide effort to achieve the IPCC 1.5-degree global warming target by the end of the 21st century. To this end, the major power generation, industrial, and transportation sectors of our economies are undergoing a transformational decarbonization process enabled by renewable energy, electrification, and carbon capture, storage, and utilization. It is anticipated that half of the primary global energy usage will be supplied through renewable sources, and electrification will reduce more than 50% of carbon emissions in the industrial and transportation sectors by 2050. However, it is challenging for diesel powered marine, trucking, and aviation forms of transportation, to embrace the “renewable + electrification” scenario because of their energy intensity for long distance travel devoid of midway charging. The UofT solar fuels group and its spin-off Solistra , have been working for more than a decade on novel photocatalysts for converting CO2. to sustainable methanol, hydrogen, carbon monoxide, methane, olefins, ammonia, and urea, powered only by sunlight. Recently they have turned their attention to green dimethyl ether, a clean replacement for diesel fuel. The motivation for our work received inspiration from the interest of Ford in renewable, high energy-density, drop-in fuels, dimethyl ether DME and poly-oxymethylene ether OME. These green replacements for brown diesel can be synthesized from renewable methanol, which can be produced from biomass, municipal solid waste, and waste plastics via gasification and syngas conversion utilizing recycled CO2. With the invention of several outstanding solar methanol catalysts, the UofT solar fuel group has begun to focus some of its efforts on solar DME and OME.
 To place our work in perspective, Ford leads a North American consortium that focuses its research and development program on renewable, high energy-density fuels for diesel engines. Global renewable energy sectors, academia and research institutions in North America and Europe are developing drop-in DME automotive fuels produced from various renewable feedstocks. We are excited that the UofT solar fuels group in partnership with UofT reactor engineering and design wing at CBBP has been and will continue to contribute to this effort by exploring the direct solar powered conversion of CO2 to methanol, DME, and OME.
To place our work in perspective, Ford leads a North American consortium that focuses its research and development program on renewable, high energy-density fuels for diesel engines. Global renewable energy sectors, academia and research institutions in North America and Europe are developing drop-in DME automotive fuels produced from various renewable feedstocks. We are excited that the UofT solar fuels group in partnership with UofT reactor engineering and design wing at CBBP has been and will continue to contribute to this effort by exploring the direct solar powered conversion of CO2 to methanol, DME, and OME.
April 23, 2021: Congratulations Alex Tavasoli,graduate student in the solar fuels group, and CEO of Solistra on being selected as a Clean50 award winner!

see web site for details: Clean50.
March 16, 2021: DIY Eco-Friendly Fertilizer

It is well-documented that around 0.00470757486 kg active nitrogen should be treated into per square meter soils to nourish the farms and gardens. Meanwhile do you know that each bolt of lightning in a thunderstorm converts about 7 kg of nitrogen into active nitrates? For sure human beings cannot rely on lightning to ease hunger but we can expect a solar powered lightning-mimic technique—the plasma, where a flow of gaseous N2 and O2 are electrically excited causing them to react to produce mainly NO in an arc reactor. This is not scientific fiction but is being reduced to practice by a company named VitalFluid. See full story at Advanced Science News..
March 15, 2021:Congratulations to Pavani, Geoff, Wei and co-authors on their publication in Science Advances!
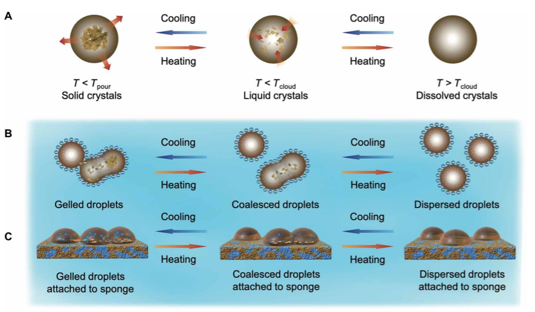
Oil spill happens occasionally and its cleanup is usually challenging especially at low temperatures. The paraffin wax in crude oil would crystallise to form thicker surface layer and block the liquid oil droplet inside, which decreases the capability of advanced disposal methods such as absorption by sponges. In this paper, Pavani, Wei and coauthors effectively resolved this challenge by coating a polyester polyurethane sponge with nanosilicon capped with paraffin-like, octadecyl ligands, achieving up to 99% absorption of oil from water at low temperatures. See full story at Science Advances..
February 19, 2021: Congratulations to Yangfan, Geoff and co-authors on their publication featured in in Cell Reports Physical Science!
Cell Reports Physical Science is a young journal from Cell Press which is dedicated to showcase high-quality, cutting-edge research from across the physical sciences. Recently, a perspective “Perovskite, the Chameleon CO2 Photocatalyst” by Yangfan, Geoff and coauthors was accepted and featured in the journal in a collection of Reviews and Perspectives — Spring 2021”. . In this paper, the tuning of compositions, structures, and forms of a famous material, the perovskite, is highlighted to achieve champion CO2 photocatalyst, which could be guided through the human intelligence, experiential learning, artificial intelligence and machine learning. See full story at Cell Reports Physical Science.
February 10, 2021: Congratulations to Joel and co-authors on their publication in Nature Communications!
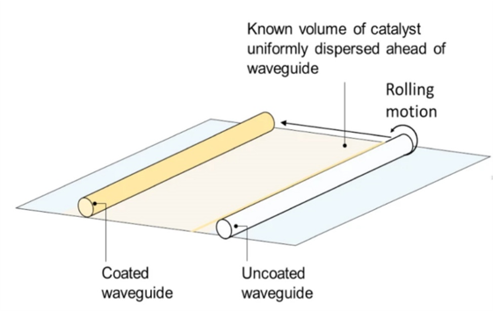
Photoelectrons could initiate excited-state chemistry to enable low-temperature reactions which are kinetically prohibited at ground state. Triggering more photoelectrons is considered as the key to implement solar advantage for facile catalysis, which is usually realized via concentrating photo-intensity. However, this method suffers from the photo-saturation effect when the light intensity reaches a threshold. In this paper, Joel and co-authors demonstrate a counter-intuitive waveguide strategy to surmount the photo saturation of indium oxide catalyst by distributing, instead of concentrating, the light intensity. See full story at Nature Communications.
January 12, 2021: Congratulations to Alex Tavasoli and coauthors on their publication in EES!
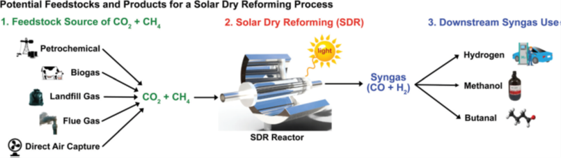
Transforming the greenhouse gas into fuels via sunlight has attracted great interests recently, but where are we and where should we go for a solar fuel refinery? To answer this question, Alex, who is the Co-founder and CEO of the solar fuel spinoff Solistra, analyzes the remaining technical challenges associated with the commercial application for dry reforming with coauthors. It is discovered that many photocatalysts reported in literatures have activity merits high enough for potential implementations while major challenge lies in chemical engineering such as the design of photoreactor. See full article at EES.
January 8, 2021: CO2 photocatalysis sees the light of day

Recently, Dimensional Energy, a spin-off company from Cornell University, has designed, built, and tested, a pilot-scale photoreactor for making chemicals using just carbon dioxide and sunlight, no additional heating or electricity is needed. As shown in the photograph, the setup consists of a tracking system, which collects and concentrates sunlight to the wave-guide reactor where the CO2 is catalytically converted to carbon monoxide or methanol by a In2O3-xOHy catalyst. This pilot reactor is now tested at a CO2 emitting coal-fired power-plant located at Gillette, Wyoming, USA to compete for the prestigious Carbon Xprize, a step closer to the vision of the solar fuel refinery. See full story at Advanced Science News.
December 24, 2020: Congratulations Dr. Mireille Ghoussoub and Professor Geoffrey Ozin, The Story of CO2 has been named in The Hill Times' List of 100 Best Books in 2020!

See the list at The Hill Times.
December 23, 2020: Congratulations Alex Tavasoli for being featured on The Chemical Engineer website!

Read the feature at The Chemical Engineer.
December 23, 2020: Playing ball with the Haber–Bosch process
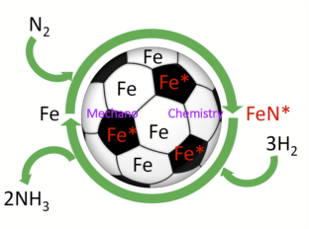
In this Advanced Science News release, a mechanochemical (ball-milling) alternative to the fossil-fuel driven Haber-Bosch process is highlighted, based on work recently reported in Nature Nanotechnology. See full article at Advanced Science News.
December 08, 2020: The Book, The Story of CO2, by Professor Geoffrey Ozin and PhD Mireille Ghoussoub is now available!

It is with great excitement and delight that we wish to announce the The Story of CO2: Big Ideas for a Small Molecule published by University of Toronto Press in November 2020 is now available for order! UofT Press.
About the book
The climate crisis requires that we drastically reduce carbon dioxide emissions across all sectors of society. The Story of CO2 contributes to this vital conversation by highlighting the cutting-edge science and emerging technologies – a number of which are already commercially available – that can transform carbon dioxide into a myriad of products such as feedstock chemicals, polymers, pharmaceuticals, and fuels. This approach allows us to reconsider CO2 as a resource, and to add "carbon capture and use" to our other tools in the fight against catastrophic climate change. See also an introduction and story behind by Faculty of Science and Art, UofT, the Story of CO2 describes a groundbreaking solution to the climate crisis.
December 08, 2020: Congratulations to Tingjiang Yan and co-authors on their publication in Nature Communications!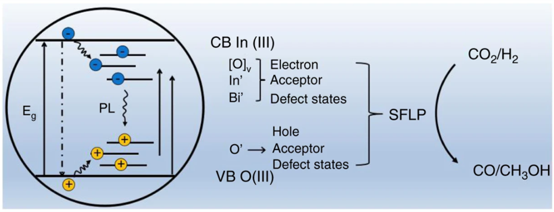
In their work, Yan et al. report an atomically-precise single-atom Bi doping method to modify the photochemistry of surface frustrated Lewis pairs (SFLP), where the isomorphic replacement of the Lewis acidic site In3+ ions in In2O3 by single-site Bi3+ ions greatly increased the efficiencies for solar absorption and charge-separation. This subtle materials chemical engineering strategy remarkably increases the photothermal CO2RR activity by three orders of magnitude.°C. See full article at Nature Communications.
November 25, 2020: Climate 21: Welcome back to a future world of green energy

This year has witnessed that a confinement of civil society did reduce anthropogenic CO2 emissions but the magnitude of the effect was not much discernible from natural annual variations of atmospheric CO2. To fully mobilize on climate change and the interlocking crises we face, it is necessary to make a multilateral, comprehensive, speedy, and effective change to a green energy infrastructure for all nations. At this very moment, the U.S. government decides back to confront the climate crisis. Geoff says it is an enormous sigh of relief for the global community to read the actionable advice expounded in the U.S. Climate 21 project.°C. See full article at Chemical Society Reviews.
November 24, 2020: Professor Geoffrey Ozin included on 5% of highly cited authors 2019 list with RSC journals, highlighting Jia Jia, and co-authors’ Chemical Society Review paper!
Congratulations to Professor Geoffrey Ozin ranking among the top 5% of highly cited authors with RSC journals in 2019, and all the authors of the 2017 review “Heterogeneous catalytic hydrogenation of CO2 by metal oxides: defect engineering - perfecting imperfection”, which is the most successful article to this regard. The success highlights the significance and impact of their work in respective fields.°C. See full article at Chemical Society Reviews.
November 13, 2020: Chimie douce: Green hydrogen

The literal translation of ‘chimie douce’, is ‘soft chemistry’. It carries connotations of reducing the extreme conditions of temperature, heat, and pressure often associated with traditional synthetic approaches for making solid-state materials to more gentle eco-friendly ambient ones. A recent Nature Energy article reports a low temperature water-splitting protocol that uses microwave power in lieu of concentrated solar energy using similar reversible metal oxide redox chemistry to the solar thermal water splitting process. The ability to “turn-down-the-heat” on these metal oxide redox cycles has been proposed to stem from microwave-induced electromagnetic field polarization, electron and oxide ion conductivity and thermalization effects. The processes work synergistically to facilitate elimination of oxygen and formation of oxygen vacancies in the metal oxide at temperatures below 250°C. See full article at Advanced Science News.
November 10th, 2020: Congratulations to Jiuli Guo and co-authors on their publication in ACS Catalysis!
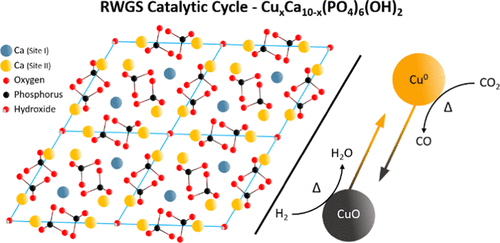
In this work, Guo et al. use abundant and nontoxic hydroxyapatite to demonstrate high activity at a low cost by replacing constituent cations with transition metals to also enable tailoring of its catalytic properties. Using this method, the facile and scalable synthesis of a copper-substituted hydroxyapatite catalyst is presented, demonstrating its high activity in the reverse water gas shift reaction. Thorough in situ characterization using X-ray absorption and Fourier transform infrared spectroscopic methods provides unrivalled insight into both the structure of the active catalyst and the speciation of reaction intermediates. It is thus shown that this copper-substituted hydroxyapatite catalyst is an exemplary candidate for use in large-scale carbon dioxide reduction systems. See full article at ACS Catalysis.
November 5th, 2020: Congratulations to Athan Tountas and co-authors on their publication in Green Chemistry!
Fossil-free and affordable syngas routes to methanol is the focus of a version 1.0 tool for investigating prospective photochemical and thermochemical heterogeneous methanol synthesis catalysts via catalytic reactor transformation. This work presents thermochemical benchmarking data with a CZA catalyst in a continuous flow reactor. These unit operational conditions allow for more efficient CO2 utilization by improving low-temperature methanol yield and reducing capital and operating costs of process equipment. See full article at Green Chemistry.
The authors wish to acknowledge Dr. Chenxi Qian for the graphical art science image.
October 13th, 2020: Congratulations to Yang-Fan Xu and co-authors on their publication in Nature Communications!
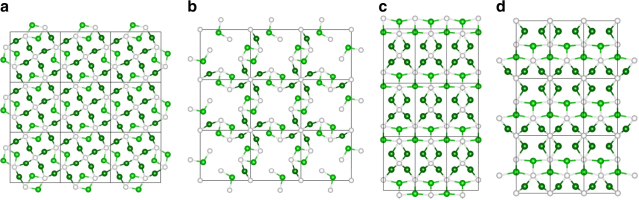
In their work, Xu et al. focus attention on an archetypal nickel phosphide, Ni12P5, with a unique surface structure based upon well-separated few-atom Ni nanoclusters. This structure allows Ni12P5 to function as an exceptionally active, selective, and stable heterogeneous catalyst for the photothermal reverse water gas shift reaction under light irradiation. The advantages of high catalytic activity and light capture are also shared by other transition metal phosphides such as Co2P. The work demonstrates how transition metal phosphides, owing to their high-performance, good stability and cost-effectiveness, offer interesting opportunities for the development of photothermal CO2 conversion technologies. See full article at Nature Communications.
October 6th, 2020: Congratulations to Dr. Qian and co-authors on their recent lithium ion battery publication in Nano Letters!
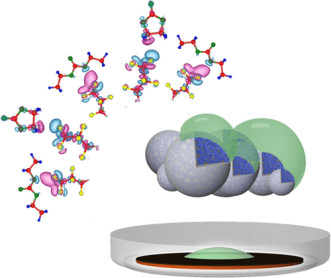
Nanostructured electrodes are among the most important candidates for high-capacity battery chemistry. However, the high surface area they possess causes serious stability, cycling and safety issues associated with the solid-electrolyte interface. In this work, Qian et al. present a completely new strategy of limiting the effective surface area by introducing an “electrolyte-phobic surface”. In this approach, the surface of the active material is coated with a chemically tethered perfluorocarbon that provides it with a unique nonwetting behavior making it impervious to the electrolyte. The concept could prove to be a general strategy for minimizing the accessible surface area of high-surface-area materials in future applications in advanced batteries. See full article at Nano Letters.
September 17, 2020: What would a room temperature superconductor mean for the energy sector?
While superconductors are not considered an energy material, the energy savings arising from resistance-free transmission and distribution of electricity are potentially massive when considered on a global scale. Energy could also be saved by incorporating room temperature superconductors into electricity generating power plants, storing electrical energy as persistent currents in superconducting magnetic loops, employing magnetically levitated railways, using superconductor propulsion motors for maritime transport, and improving the energy efficiency of quantum computers with superconducting digital logic circuitry. See full article at Advanced Science News.
September 14, 2020: Imagining how “synthetic topology” could reform carbon dioxide catalysis

Solid-state physicists and materials chemists are in excellent “shape” to expand and accelerate their explorations of the science of topological materials for a wide range of possible applications. In particular, exploiting the reactivity of electrons at the surface of topological solids will prove to be of considerable interest in catalysis, whether a heterogeneous reaction is driven by heat, electricity, light or a combination thereof. See full article at Advanced Science News.
August 26, 2020: Dual aluminum-nitrogen battery that stores energy and fixes nitrogen

A recent report described an innovative rechargeable metal-nitrogen battery based on a graphene-supported palladium cathode and a polished aluminum anode interfaced with an ionic liquid electrolyte comprised of aluminum chloride/1-butyl-3-methylimidazolium chloride. Remarkably, the aluminum-nitrogen battery was demonstrated to serve the dual purpose of both storing and retrieving energy and having the ability to fix the nitrogen stream as ammonia. See full article at Advanced Science News.
August 25, 2020: What is the carbon footprint of carbon capture and utilization?

Capturing carbon dioxide and sequestering or converting it into sustainable chemicals and fuels can significantly help in mitigating emissions as we transition away from fossil fuels. But what exactly is the carbon footprint of such a process from cradle-to-grave? How much carbon dioxide feedstock needs to be captured to satisfy the demand for chemicals and fuels? And what is the energy demand of the CO2 capture and utilization processes? Geoff explores these pressing questions in his latest for Advanced Science News.
August 23, 2020: Celebrating the 77th birthday of "the father of Nanochemistry"—Geoffery Ozin, and his colorful career
To celebrate his 77th birthday, ASN reached out to the extraordinary Nanochemist at the University of Toronto, "the father of Nanochemistry", Geoffery Ozin, to discuss his colorful career, current projects, and plans for the future. The interview is summarized with the title "small materials with a big impact". See the full article at Advanced Science News.
August 5, 2020: Congratulations to Xiaoliang Yan and co-authors on the publication of their communication article in Small
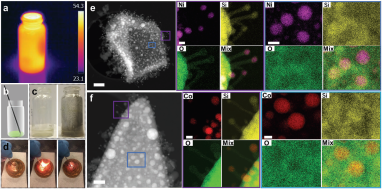
1D silicon‐based nanomaterials, renowned for their unique chemical and physical properties, have enabled the development of numerous advanced materials and biomedical technologies. In this work, the authors demonstrate a flash solid–solid (FSS) process for the synthesis of silicon oxide nanorods that can be completed within seconds. The innovative features of this FSS process include its simplicity, speed, and exclusive use of solid precursors, comprising hydrogen‐terminated silicon nanosheets and a metal nitrate catalyst. See full article at Small.
August 4, 2020: Congratulations to Professor Ozin and co-authors on their article published in Energy & Environmental Science
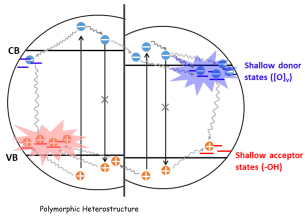
Tailoring the performance of a photocatalyst by design is challenge in the field of renewable synthetic fuels. In this work, the authors demonstrate how polymorphic heterostructures comprised of two indium oxide based photocatalysts, with distinct structures yet continuously adjustable fractions of the same composition, enable optimization of the activity and selectivity of CO2 hydrogenation to CO and CH3OH. Interfaces formed between cubic and rhombohedral polymorphs with distinct electronic band structures, vacancies, and defects enable the charge generation, separation, and lifetimes of photogenerated electron-hole pairs to be finely tuned. See full article at Energy and Environmental Science.
July 28th, 2020: Congratulations Yuchan Dong and co-authors on their recently published Chemical Society Review paper, celebrating Geoff's 77th birthday and winning the front cover display of one of the highest impact factor journals in the field of chemistry
Powering the planet with sunlight-driven CO2 chemistry is an especially attractive approach for sustainable development. In this Tutorial Review, Yuchan Dong and co-authors highlight the multidisciplinary character of photocatalytic CO2 reduction studies from the perspective of materials chemistry, science and engineering, computational modelling, reactor engineering to process development. It provides a full picture of all the essential components for scaling laboratory research to pilot demonstration to implementation in industry, one-step closer to the vision of the solar CO2 refinery. See full article at Chemical Society Review.
July 11th, 2020: Congratulations Young Li and co-authors on their recently published paper
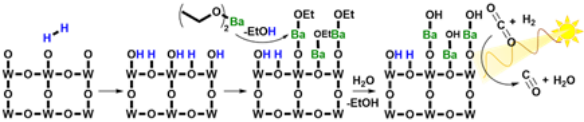
In this work, Young Li and co-authors report how a conformal coating of the well-documented catalyst promoter barium on the surface of palladium-decorated tungsten oxide nanowires was developed using a solution‐phase atomic layer deposition process. At just 0.2 atomic percent barium, a significant promotion of the light‐assisted activity of the reverse water gas shirt reaction was observed. See full article at Chemistry-A European Journal.
July 10th, 2020: The three colors of hydrogen
Everybody knows that H2, the archetype diatomic molecule, is a colorless gas. In the field of renewable energy however, hydrogen gas is now considered as either “grey”, “blue”, or “green”. This color code descriptor has arisen in order to differentiate hydrogen according to its source, in particular to distinguish hydrogen derived from fossil sources from that derived from renewable energy. Today, exciting steps are being made towards realizing this vision of a hydrogen-powered zero emission infrastructure. See full article at Advanced Science News.
July 8th, 2020: Congratulations to the authors of “Solution–Liquid–Solid Growth and Catalytic Applications of Silica Nanorod Arrays”
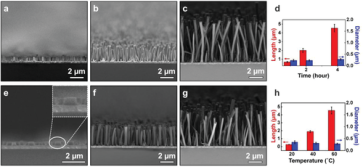
As an analogue to the vapor–liquid–solid process, the solution–liquid–solid (SLS) method offers a mild solution‐phase route to colloidal 1D nanostructures with controlled sizes, compositions, and properties. Direct growth of 1D nanostructure arrays through SLS processes remains in its infancy. This study shows that SLS processes are also suitable for the growth of nanorod arrays on the substrate. See full article at Advanced Science.
June 25th, 2020: Are we dehumanizing chemistry?

A new paradigm is emerging in the way materials are discovered on a computer and made in the laboratory. Computational searches can now be designed to aid the experimental discovery stage of a new material with a designated set of properties considered ideal for a particular application. On-line robot chemists can reduce these materials discovery pathways to practice thereby completing an accelerated materials discovery process. Is this what the future of chemistry holds for its creative inquisitive practitioners? See full article at Advanced Science News.
June 17th, 2020: What ever happened to combinatorial materials discovery?
It is not exactly clear whether the exciting potential of combinatorial chemistry research and development in the 1990s led to new products. After about a decade or so of intense activity and excitement, the field seemed to fade away. Today, synthetic chemists, artificial intelligence, machine learning, big data, supercomputers, computer simulations, and robotics appeared on our radar. At breakneck speed they started to take off where combinatorial chemistry had left off and the self-driving chemistry laboratory and artificial chemist made their debut. It will be very interesting to see if the “next industrial revolution” will be spawned by this new wave of computational materials research. See full article at Advanced Science News.
June 12th, 2020: Getting a charge out of liquid metal batteries

A novel concept in reversible chemical to electrical energy storage is the liquid metal battery, in which a metal anode, metal cathode, and salt electrolyte are all in the liquid phase. Although the liquid metal battery is heavy, it could be more suitable for large scale electricity storage. In this article, Geoffrey Ozin explains the operation, advantages, and potential impact of liquid metal battery technology. See full article at Advanced Science News.
June 4th, 2020: Flying high on carbon dioxide: Decarbonizing aviation

In a pilot plant demonstration of the EU-project Sun-to-Liquid, a solar thermochemical process has been successfully scaled to a solar tower in Madrid, leading to the first-ever production facility for making synthetic kerosene from water and carbon using concentrated solar energy. This pioneering development is at the foundation of the partnership of the two ETH spinoffs, Climeworks and Synhelion, with Lufthansa who are working to commercialize and implement the technologies at an industrial scale for direct air capture and solar fuels production. See full article at Advanced Science News.
May 26th, 2020: Online learning in a pandemic: A perspective

The whole world is being challenged momentarily. Everyday routines and habits have come to a halt and we are getting used to new daily schedules. School is now online for many students worldwide, and the idea and introduction of online schooling was quickly sprung upon us. It has been very difficult to get used to this “new normal” system and operate not only as a student, but equally a teacher. See full article at Advanced Science News.
Monday, May 18th, 2020: Congratulations to Lu Wang and co-authors on their publication in Nature Communations
Black indium oxide comprises amorphous non-stoichiometric domains of In2O3-x on a core of crystalline stoichiometric In2O3. In their paper, Lu Wang and co-authors demonstrate black indium to have a 100% selectivity towards the hydrogenation of CO2 to CO with a turnover frequency of 2.44 s−1. Their result shows that based on its activity, selectivity and stability, black indium oxide outperforms all known indium-oxide-based photocatalysts.
Monday, May 11th, 2020: Cobalt Plasmonic Superstructures Enable Almost 100% Broadband Photon Efficient CO2 Photocatalysis
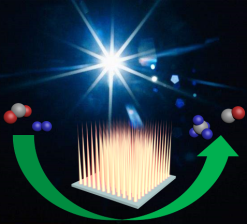
Congratulations to the authors of “Cobalt Plasmonic Superstructures Enable Almost 100% Broadband Photon Efficient CO2 Photocatalysis” for having their paper recently accepted to Advanced Materials. Their work demonstrates photothermal hydrogenation of CO2 using a plasmonic superstructure, consisting of an array of nanoneedles, to enhance the absorption of sunlight. See full paper at Advanced Materials.
Tuesday, May 5th, 2020: Thoughts on materials discovery at the human-machine interface
The emergence of artificial intelligence, machine learning, and robotic automation in the field of materials discovery can really take-off where combinatorial materials chemistry left-off and positively disrupt the field of materials chemistry as we know it today. In this opinion editorial, Geoff explores how “energy materials discovery” is used as a training ground to explore this question and compare and contrast the old way with the new way of operating in an attempt to discover how humans and machines can work harmoniously together to the benefit of each. See full article at Advanced Science News.
Friday, May 1st, 2020: Congratulations to Athan Tountas, Wei Sun, and co-authors on the success of their papers!
Congratulations to all the authors of the 2019 review, “Towards Solar Methanol: Past, Present and Future" and the 2019 paper, “Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst". Their work is among the top downloaded articles featured on Wiley Advanced Science. The success of these papers among readers highlights their significance and impact in their respective fields.
Thursday, April 23rd, 2020: Congratulations Xian-Wei Lv, Lu Wang, and co-authors on your recently accepted paper!
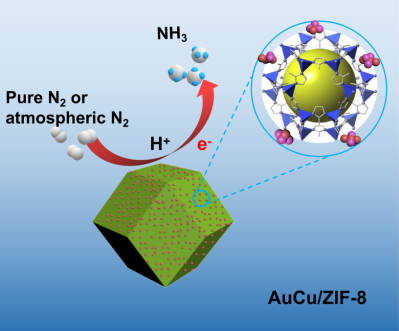
Nitrogen fixation using electrochemistry presents an environmentally-sound alternative to the energy-intensive Haber-Bosch process. Using air as the feed stock would represent an important step towards future industrialization of green ammonia. In their paper, ZIF-supported AuCu Nanoalloy for Ammonia Electrosynthesis from Nitrogen and Thin Air, Lv, Wang, and co-authors report a gold-copper nanoalloy-decorated zeolitic imidazolate framework that exhibits a high ammonia yield, remarkable efficiency, long-term stability and pH independence, demonstrating the technological viability of producing ammonia from thin air.
Tuesday, April 21st, 2020: Is your work not novel enough?

Global scientific output roughly doubles every nine years and editors are left facing the tsunami of publications seeking homes in elite journals. The situation has created the perfect storm for biased selection criteria – whether intentional or unintential - at the peer review gate, putting many researchers at a disadvantage. Is it time to rethink our values in higher education and place real science and social creativity as the key criteria in this sector rather than productivity and profit? Perhaps the time has come to rethink how and where we showcase our breakthroughs. See full story at Advanced Science News.
Monday, April 20th, 2020: Upcoming Book Release

It is with great excitement that we wish to announce the upcoming release of The Story of CO2: Big Ideas for a Small Molecule by Prof. Geoffrey Ozin and PhD candidate Mireille Ghoussoub. The Story of CO2 is anticipated for release in October 2020 by University of Toronto Press. Now available for pre-order!
About the book: The climate crisis requires that we drastically reduce carbon dioxide emissions across all sectors of society. The Story of CO2 contributes to this vital conversation by highlighting the cutting-edge science and emerging technologies – a number of which are already commercially available – that can transform carbon dioxide into a myriad of products such as feedstock chemicals, polymers, pharmaceuticals, and fuels. This approach allows us to reconsider CO2 as a resource, and to add "carbon capture and use" to our other tools in the fight against catastrophic climate change.
Friday, March 27th, 2020: Seeking connection through art in a pandemic
With the COVID-19 pandemic threatening public health and rapidly spreading around the world, science communication has never been more timely. In their article, Dr. Geoffrey Ozin and artist Todd Siler engage in cross-disciplinary collaboration to speak to what is currently happening around the globe. The artwork displayed in this article intimates how a chance natural event can create a widespread human catastrophe; one that involves a molecular biological assembly, a nano-bio-scale material, as exemplified by COVID-19. See full story at Advanced Science News.
Monday, March 10th, 2020: Prof-Bot: The autonomous chemistry professor

Are the wide-ranging tasks and the vast repertoire of skills of the university teaching professor replaceable by a robot? With recent developments in supercomputer power, big data, artificial intelligence, and machine learning, it is not beyond the realm of possibility that with all-knowledge endowed robot educators and robot assistants could rise to match or even outperform professors and teaching assistants in the theater of higher learning. See full story at Advanced Science News.
February 28th, 2020: Autonomous Chemical Synthesis

The self-driving chemistry laboratory has now made its debut, thanks to advances in artificial intelligence, machine learning, big data, supercomputers, and robotics. The possibilities for chemistry now seem boundless, only constrained by the imagination of humans and/or robots who are going to have to learn to live, work, and play together. As self-driving laboratories further develop, it is worth asking how this will impact the nature of chemistry research and direct innovation in the chemical sciences in the long-term. See full story at Advanced Science News.
February 18th, 2020: Absolute zero: A counter-intuitive energy transition

Net zero emissions by 2050 was a target set by the Intergovernmental Panel on Climate Change to increase our changes of limiting global temperature rise to under 1.5 °C. But how feasible is this in practice? Absolute Zero, a recent report laid out by British researchers, argues that the 2050 target can only be achieved via large-scale implementation of tried and trusted technologies, such as solar, wind, and geothermal power. Newer, riskier approaches, such as those based on hydrogen, bioenergy, and carbon capture, will likely require longer than 30 years to develop, deploy, and scale, and therefore may not be suitable for meeting short-term emission targets. See full story at Advanced Science News.
January 28th, 2020: Congratulations to Mireille Ghoussoub on winning 1st Place in the 3MT Competition at the WISE National Conference!

PhD Candidate Mireille Ghoussoub placed first in the Three Minute Thesis Competition at the Women in Science and Engineering (WISE) National Conference over the weekend. Her research focuses on merging experimental and computational characterization tools to study catalysts for converting carbon dioxide into methanol.
The WISE National Conference serves as a catalyst to inspire and empower individuals to pursue their passions, broaden their horizons, and form meaningful connections. The conference brings together delegates from all across Canada to share ideas and become inspired over the course of a two-day event dedicated to professional and personal growth, featuring inspirational leaders from a wide range of STEM fields, as well as workshops, case competitions, and career fairs.
January 20th, 2020: Financing Net Zero

Making financial policy a centerpiece for governments, businesses, and partnerships is a critical piece to achieving a net-zero economy. A challenge in this, however, is to build comprehensive transparency by all climate stakeholders on climate change, risk management, and investment to help companies deal with the financial stress associated with the potentially disruptive effects of a global energy transition. See full story at Advanced Science News.
January 10th, 2020: Nano-silicon Samurai

In addition to being an important element in living organisms, silicon is by far the most widely used of all semiconductor materials. Most recently, however, nano-silicon has been demonstrated to be an attractive tool in the field of environmental remediation, specifically when it comes to cleaning up oil spills from underwater drilling and hydraulic fracking. Here, the notable advance is that surface-engineered porous nano-silicon sponges demonstrate a strong affinity for emulsified oil in seawater. See full story at Advanced Science News.
December 20th, 2019: A Fossil Fuel-Free Industrial Revolution

While coal combustion energized James Watt’s steam engine, it is seldom acknowledged that other sources of energy were also known at the time. As recently as the late 1800s, electricity could be generated for a myriad of applications through the use of waterwheels and windmills. The fact that these technologies were contemporaries of the now-dominant internal combustion engine suggests that non-fossil energy technologies were equally poised to become globally widespread. But could the technological revolution of the 18th and 19th centuries have happened without the use of fossil fuels and the rampant burning-down of forests? See full story at Advanced Science News.
December 16th, 2019: Congratulations to Dr. Pavani Cherukupally, Wei Sun and co-authors on your publication in Nature Sustainability!
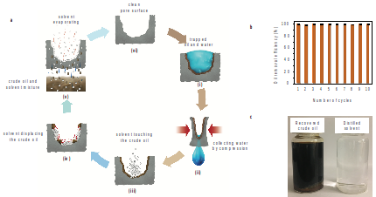
In their paper, “Surface-engineered sponges for recovery of crude oil microdroplets from wastewater”, Cherukupally and Sun et al. report an innovative surface engineered sponge that combines surface chemistry, pH-responsive surface charge, and micro-nano roughness that allows for upwards of 92% of crude oil to be adsorbed under ambient conditions. Their work constitutes a new framework for developing surface engineered sponges that addresses the variable pH-responsive wetting behaviour of crude oil and further demonstrates the environmental remediation potential of adsorptive recovery through sponge technology. See full story at Nature Sustainability.
December 4th, 2019: Sunshine Not Moonshine – Happy Hour with Carbon Dioxide

Many novel chemistry and engineering approaches now exist that enable CO2 to be recycled into a cornucopia of products ranging from plastics and pharmaceuticals, to fertilizers and concrete, and even aviation and clean diesel fuels. One particularly innovative, and perhaps less conventional solution, to have recently emerged is an eco-friendly vodka made from CO2 and H2O. This concept has been realized through The Air Company, a legal distillery and start-up company located and operating out of Brooklyn, run by two young entrepreneurs, Stafford Sheehan and Gregory Constantine. See full article at Advanced Science News.
November 25th: Congratulations to Dr. Hong Wang and co-authors on their Advanced Science Cover!

Congratulations to Dr. Hong Wang and co-authors on having their article, “CO2 Photoreduction: Heterostructure Engineering of a Reverse Water Gas Shift Photocatalyst” featured on the cover of Advanced Science Online. The image is the creation of artist and researcher, Dr. Chenxi Qian. See full article at Advanced Science Online.
October 25th: Gigawatt Electricity Storage Using Water and Rocks

The dominant technology for the large-scale storage and retrieval of electricity is pumped hydro - electrical energy. In this approach, electricity is converted to gravitational potential energy by moving water uphill and is then retrieved by allowing the water to flow downhill through a turbine. However, a new concept in gravity storage eliminates the need for hills and simply uses water pumps to hydraulically lift massive rocks in an underground shaft. The acquired potential energy is reclaimed as electricity by discharging the water under pressure though a turbine. See full article at Advanced Science News.
October 17th, 2019: Beyond Haber-Bosch: Non-Equilibrium Photocatalysis
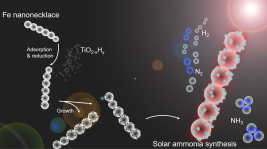
A recent report in which the thermodynamic equilibrium limit of the Haber-Bosch synthesis of ammonia was, for the first time, surmounted by the action of light, and could change the prevailing view of what is possible and not possible in the field of catalysis. A hetero-nanostructured photocatalyst, Fe-TiO2-xHy, which contains both a high and low-temperature site in a single structure has been demonstrated to overcome the thermodynamic equilibrium limit of the ammonia synthesis. This is possible because the exothermic ammonia synthesis process is thermodynamically favored though kinetically sluggish at low temperature; however, the exact opposite prevails at high temperature. See full article at Advanced Science News.
October 14th, 2019: Climate Change Will Require Heavy Lifting
As the global hunger for electricity grows and the transition to solar and wind accelerates, electricity storage capacity is urgently needed to handle the challenges of scale and intermittency. Concrete solutions are needed to solve the large-scale electricity storage problem for both daily and seasonal applications, and it’s going to require some heavy lifting. A new generation of gravity batteries have emerged based on the lifting and lowering massive concrete weights. The solution may prove a viable option for storing and releasing grid scale electrical energy over periods as short as seconds to as long as months, which would represent a significant step towards renewable energy utilization. See full story at Advanced Science News.
October 9th, 2019: A Whale of a Solution to Climate Change

Strategies to enhance the carbon capacity of the terrestrial and oceanic sinks tend to focus on landscapes – forest canopy, soil composition, and sea water chemistry. However, we must not neglect the role of animals in maintaining the natural carbon cycle. As it turns out, whales have the capacity to absorb enormous amounts of atmospheric carbon dioxide (CO2). Estimates place the carbon sequestering capacity of a whale to be similar to around 1000 trees with an average whale capturing up to 33 tons of CO2 over its 60-year lifetime, centuries. Efforts to re-establish whale populations, together with large scale reforestation, therefore offer a surprinsingly impactful solution to meeting global emission targets. See full article at Advanced Science News.
October 4th, 2019: Congratulations to Hong Wang et al. on their publication in Advanced Science, “Heterostructure Engineering of a Reverse Water Gas Shift Photocatalyst”
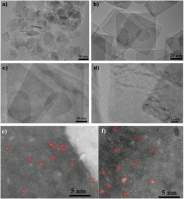
The paper describes a heterostructure engineering strategy that enables the gas-phase, photocatalytic, heterogeneous hydrogenation of CO2 to CO with high performance metrics. The catalyst is comprised of indium oxide nanocrystals (In2O3-x(OH)y) nucleated and grown on the surface of niobium pentoxide nanorods. Materials characterization demonstrates that the Nb2O5 support in the In2O3-x(OH)y@Nb2O5 heterostructure increases the number of oxygen vacancies and lengthens the excited state charge carrier lifetimes in the attached In2O3-x(OH)y nanocrystals, which results in a 44-fold higher conversion rate than pristine In2O3-x(OH)y selective conversion of CO2 to CO as well as long-term operational stability. Overall, the results of this study bode well for the general applicability of the heterostructure engineering approach for optimizing the performance of photocatalytic heterogeneous CO2 conversion reactions. See full article at Advanced Science.
September 20th, 2019: SF6 Worries - The Most Potent and Persistent Greenhouse Gas

It is not well known, but the most potent greenhouse gas is, surprisingly, neither carbon dioxide nor methane, but a colorless, odorless, and inert gas known as sulfur hexafluoride (SF6). With a global warming potential 23,900 times that of CO2 and being synthetic in nature (it is not absorbed on destroyed naturally), rising SF>6 concentrations are of major concern. Currently, electrical utilities and equipment are responsible for consuming 80% of the 10 000 tons of SF6 produced every year, an amount which is growing with the increasing global production and demand for renewable forms of energy, such as wind and solar. Can chemists and engineers rise to the challenge of solving the looming SF6 problem? See full article at Advanced Science News.
September 16th, 2019: Congratulations to Young and co-authors on their article in JACS
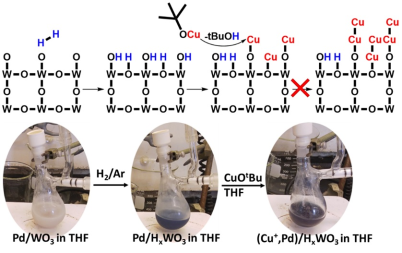
Atomically precise heterostrucutures present chemically interesting active sites for catalysis but are often expensive and/or challenging to synthesize. In this article, we report a synthetic strategy to conformally coating Cu atoms onto the surface of Pd/HyWO3-x by anchoring Cu(I)OtBu to the Brønsted acidic protons of the bronze. It was observed that just 0.2 at.% of Cu was able to increase the catalytic performance of CO2 hydrogenation to CO by 500%. This metal anchoring method enables atom precise modification of the surfaces of metal oxide nanomaterials for catalytic applications, circumventing the need for complex and expensive atomic layer deposition processes. See full article at Journal of the American Chemical Society.
September 9th, 2019: Congratulations to Lili Wan, Wei Sun, & co-authors on their article in Nature Catalysis
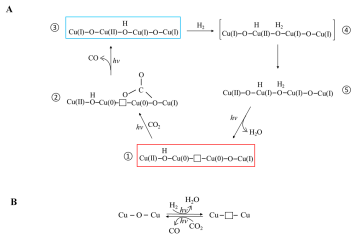
A long-standing challenge in the field of CO2 utilization is how to stabilize Cu2O, an earth-abundant, non-toxic, low-cost (photo)catalyst that can facilitate reduction of CO2 to CO against the irreversible redox disproportionation Cu2O → Cu + CuO, responsible for its instability.
Lili Wan and Wei Sun and coworkers in the Ozin group have solved this problem as reported in their Nature Catalysis paper, by modifying the surface of Cu2O nanocubes with a mixed valence surface frustrated Lewis pair (SFLP), Cu(I,II) ●● OH, which serves to eliminate the redox disproportionation. As depicted in the illustration, H2 undergoes heterolytic dissociation on the SFLP, water is eliminated to create an [O] vacancy and Cu(I) is reduced to Cu(0). Adsorption of CO2 at the [O] vacancy site drives the conversion to CO thereby completing the photocatalytic RWGS reaction cycle. See full article at Nature Catalysis online.
August 13th, 2019: Congratulations to Dr. Zaiyong Jiang et al. on their paper, "Building a Bridge from Papermaking to Solar Fuels"

Everybody knows the leaf makes carbohydrates and the trunk makes paper. But did you know that waste from the paper making process can make fuel from carbon dioxide, water and sunlight? Black liquor, an industrial waste product of papermaking, is primarily used as a low‐grade combustible energy source. Despite its high lignin content, the potential utility of black liquor as a feedstock in products manufacturing, remains to be exploited. In this paper, black liquor is demonstrated to function as a primary feed‐stock for synthesizing graphene quantum dots that exhibit both up‐conversion and photoluminescence when excited using visible/near‐infrared radiation, enabling solar‐powered generation of H2 from H2O, and CO from H2O–CO2, using broadband solar radiation. See full article at Angewandte Chemie.
August 7th, 2019: Congratulations to Zaiyong Jiang, Wei Sun, & co-authors on their article in Advanced Science
In their paper, “Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst”, Jiang, Sun, and co-authors report a serendipitous living photodeposition method can make atomically dispersed Cu immobilized on ultrathin TiO2 nanosheets, which can photocatalytically reduce an aqueous solution of CO2 to CO and in the process can be recycled in a straightforward procedure on becoming oxidatively deactivated. See full article at Advanced Science.
July 17th, 2019: Fundamentals and Applications of Photocatalytic CO2 Methanation
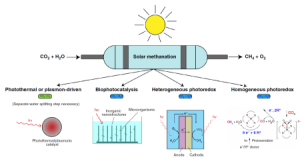
Light-driven methanation of CO2 holds great promise to close the carbon cycle and store and transport intermittent solar energy in the form of the chemical bonds of synthetic, “solar” methane. In their Review article published in Nature Communications, Geoffrey Ozin, Uli Ulmer and co-authors critically appraise the latest scientific discoveries in the field of photocatalytic CO2 methanation. The photocatalytic methanation schemes explored include photothermal, plasmonic, biophoto, heterogeneous and homogeneous photoredox methanation. Photocatalytic CO2 methanation could eventually replace fossil CH4 if implemented at a large scale.
July 5th, 2019: Challenges of Electrifying Heterogeneous Catalysis
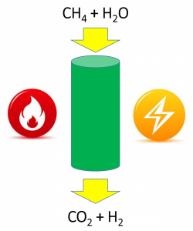
How can we replace the fossil-fuel-derived heat that conventionally drives chemical processes with an emissions-free alternative? The current standard in industrial-scale heterogeneous catalytic processes, such as the production of ammonia and hydrogen, is to use heat supplied by the combustion of natural gas. Heat can alternatively be supplied from non-fossil sources, such as renewable electricity; however, assessing the net impact on carbon emissions of electricity-based processes remains non-trivial. Read full article at Advanced Science News.
June 21th, 2019: Electrochemical Carbon Dioxide Reduction in Supercritical Carbon Dioxide is Cool
At standard temperature and pressure, CO2 exists as a gas. On cooling to -78.5 °C, it becomes a solid called dry ice, which is a common refrigerant. At a critical temperature of 31.1 °C and pressure 72.9 atmospheres, however, CO2 becomes a supercritical fluid with properties intermediate to a gas and liquid. In this form, CO2 fills a containment vessel and exhibits a low viscosity reminiscent of a gas but retains the high density of a liquid. It turns out that supercritical CO2 also has rather appealing properties as both solvent and reagent in the electrochemical reduction of CO2 to a variety of products. Read full article at Advanced Science News.
June 13th, 2019: Congratulations to Dr. Xiaoliang Yan and co-authors on their paper, “Nickel@Siloxene catalytic nanosheets for high-performance CO2 methanation”
Two-dimensional materials hold great potential as catalysts for the heterogeneous conversion of CO2 to synthetic fuels and chemicals. In this paper, Yan et al. demonstrate the performance of nickel@siloxene to be highly sensitive to the nickel component being located either on the interior or exterior of adjacent siloxene nanosheets. Control over the location of nickel is achieved by employing the terminal groups of siloxene and varying the solvent used during its nucleation and growth, which ultimately determines the distinct reaction intermediates and pathways for the catalytic CO2 methanation. A CO2 methanation rate of 100 mmol gNi−1 h−1 is achieved with over 90% selectivity when nickel resides specifically between the sheets of siloxene.. See full paper at Nature Communications.
June 10th, 2019: Congratulations to Dr. Tingjiang Yan and co-authors on their paper, “Polymorph selection towards photocatalytic gaseous CO2 hydrogenation”
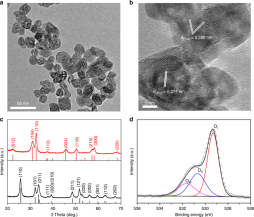
Titanium dioxide is the only known material that can enable gas-phase CO2 photocatalysis in its anatase and rutile polymorphic forms. In their paper, however, Dr. Yan and co-authors demonstrate that the lesser known rhombohedral polymorph of indium sesquioxide, like its well-documented cubic polymorph, can act as a CO2 hydrogenation photocatalyst with the ability to produce CH3OH and CO. See full paper at Nature Communications.
June 5th, 2019: Electricity-free Renewable Hydrogen
A recent paper published in the Proceedings of the National Academy of Science has reported that applying carbon capture and utilization (CCU) to manufacture the top 20 commodity chemicals could mitigate up to of 3.5 gigatonnes of carbon emmisions annually, equivalent of nearly 10% of the emissions released in 2018. The study found CCU’s potential to be contingent on whether the vast amount of electricity it would require could actually be provided given the limited renewable electricity infrastructure that currently exists. How do we decide which energy-consuming processes should be made priority? Read full article at Advanced Science News.
May 21st, 2019: Global Carbon Dioxide Cooling

It may come as a surprise, but carbon dioxide, the infamous greenhouse gas driving climate change, is also a leading contender in the replacement of hydrofluorocarbons in the next-generation of “green” refrigeration systems. It offers many advantages over second and third generation refrigerants, including higher volumetric cooling capacity, lower operating temperatures, non-flammability, reduced operating costs, and lower global warming potential. See full article at Advanced Science News.
May 14th, 2019: Congratulations to Dr. Lu Wang and co-authors on their paper in Angewandte Chemie!
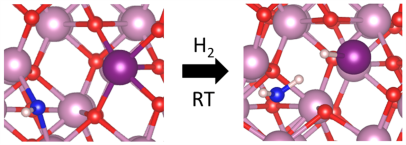
Surface Frustrated Lewis Pairs (SFLPs) have been implicated in the gas‐phase heterogeneous (photo)catalytic hydrogenation of CO2 via the cubic form of hydroxylated indium oxide. In their paper, Dr. Wang and co-authors report the room temperature dissociation of molecular hydrogen via SFLPs on the rhombodral form of the catalyst, which is shown to favour the heterolysis over the homolysis reaction pathway. See full article at Angewandte Chemie.
May 3rd, 2019: Is Air Conditioning Cool?

When one thinks of the major sources of anthropogenic emissions entering the earth’s atmophsere, chances are that air conditioning (AC) isn’t the first to come to mind. However, the carbon footprint of AC systems is far from negligeable: they are expected to contribute an additional 167 gigatonnes of CO2 by 2050. But what if AC systems could be re-designed to provide an opportunity to capture CO2 from the air? Global adoption of on-site conversion of CO2 from AC systems into chemicals and fuels could have a key role in addressing global climate change. Read the full article at Advanced Science News.
May 1st, 2019: Crowd oil not crude oil

Congratulations to authors Prof. Roland Dittmeyer, Michael Klumpp, Paul Kant, and Prof. Geoffrey Ozin on the release of their Nature Communications article, “Crowd oil not crude oil”. The delocalized nature of climate change poses a major challenge to mitigation efforts. The authors propose retrofitting air conditioning units to convert water and carbon dioxide into fuel. The users would collect the synthetically-made oil for their personal use, or to redistribute within their community, as to encourage decentralized CO2 conversion and energy democratization. Read the full article at Nature Communications.
April 25th, 2019: Advanced Science News: “Spamming Science”

The internet has had profound effects on virtually all aspects of society. It has revolutionized every part of our lives, from the ways in which we engage and communicate, to our shopping and entertainement habits, to our media and politics. But what of the effect of the internet age on academia? As one of the earliest adopters of email communication, academics have witnessed the many ways in which the internet has shifted the nature of scientific collaborations, publishing processes, and research patterns. Have we, however, reached a point where electronic communication has become more problematic than beneficial to academic research? Read Prof. Ozin and Prof. Seferos’s take in Advanced Science News.
April 20th, 2019: Congratulations to Athan Tountas & co-authors for having their review article featured in Advanced Science’s 5 Year Anniversary Digital Issue!

On the occasion of its 5th anniversary, Advanced Science’s virtual issue collects a celebratory series of invited-only articles which showcase the outstanding achievements of leading international researchers in the field of materials science, physics and chemistry, medical and life sciences, as well as engineering. In their article, “Towards Solar Methanol: Past, Present, and Future”, Athan Tountas and co-authors provide an overview of how conventional industrial-scale fossil-enabled heterogeneous catalytic conversion of carbon monoxide and hydrogen to methanol is being challenged by more sustainable electrocatalytic, photocatalytic, biocatalytic, and solar thermal methods using carbon dioxide and water as feed-stocks. See full article at Advanced Science. Cover art courtesy of Dr. Chenxi Qian.
April 11th, 2019: Energy & Environmental Science Cover Page!

Congratulations to authors Mireille Ghoussoub, Meikun Xia, Dr. Paul Duchesne, and Prof. Dvira Segal, as well as cover artist and alumnus, Dr. Chenxi Qian, for having their recently published review article featured on the cover of Energy and Environmental Science. Photothermal catalysis is an emerging sub-discipline of heterogeneous catalysis that exploits broad absorption of the solar spectrum to stimulate a combination of thermochemical and photochemical processes, which contribute synergistically to driving catalytic reactions. It is proving an effective and promising strategy for converting CO2 to synthetic fuels. See full article at Energy & Environmental Science.
March 20th, 2019: Putting a Spin on a Carbon Photocatalysis Spin-off

Chemicals and fuels derived from CO2 and enabled by solar power have taken the research world by storm over the past decade. However, CO2-derived fuel technologies, as promising as they may appear, are still subject to the harsh economic realities of chemical engineering. Is it possible to simultaneously achieve high photonic efficiencies all whilst adhering to the logic of economies of scale? Two new start-ups, The Solistra Corporation and Dimensional Energy, have taken on this challenge and are paving the way towards a future driven by solar fuel technology. Read full story at Advanced Science News.
February 22nd, 2019: Industrial Carbon Dioxide Photoctatalysis

Where did the use of light to drive catalytic reactions all begin? Why are metal oxides a good choice for light-assisted chemical reactions? How can we design better photoreactors to integrate into the existing chemical infrastructure? Finally, what are the prospects for global industrial photocatalysis? Learn the answers to all these questions and more as the University of Toronto Solar Fuels Group shines light some of the technological challenges facing the widespread industrialization of CO2 photocatalysis. Read full story at Advanced Science News.
February 21th, 2019: Congratulations Dr. Yang-Fan Xu on being selected as a recipient of the 2019 University of Toronto, Faculty of Arts & Science Postdoctoral Fellowship Award

The Faculty of Arts and Science (FAS) Postdoctoral fellowship is a highly competitive and prestigious award designed to provide outstanding recent doctoral students advanced training in their field of study. This year the FAS received over 140 applicants. The Solar Fuels Groups is pleased to announce that Dr. Yang-Fan among the few selected recipients.
February 19th, 2019: Congratulations to Athan Tountas and Co-authors on your Solar Methanol Advanced Science Paper!

“Towards Solar Methanol: Past, Present, and Future” provides a comprehensive overview of how value-added products, notably methanol, can be produced affordably and sustainably from greenhouse gases. Harnessing light in the form of solar energy can assist is the production process in some capacity through various strategies, such as solar-thermochemical, photochemical, and photovoltaic-electrochemical. Commercially-ready technologies are compared via technoeconomic analysis, and the scalability of solar reactors is also discussed in the context of light-incorporating catalyst architectures and designs. Finally, the review offers perspective on the viability of the most promising solar methanol strategy to be applied at a global scale. Read full story at Advanced Science.
January 10th, 2019: Catalytic CO2 Reduction by Palladium Decorated Silicon Hydride Nanosheets
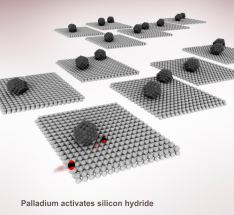
Congratulations to Wei, Chenxi, Govind, and Co-Authors on their New Years Eve Nature Catalysis publication in which they report on their discovery of how to make Silicon, the second most abundant element on earth, behave catalytically in the gas-phase heterogeneous hydrogenation of CO2 to CO, known as the Reverse Water Gas Shift Reaction. See full article at Nature Catalysis.
January 8th, 2019: Congratulations Mireille, Dvira, Meikun, and Paul on your EES article which shows how producing synthetic fuels by photothermal gas-phase heterogeneous CO2 catalysis is within our hands!
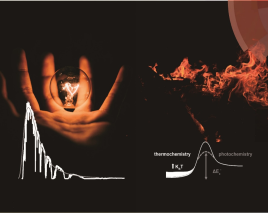
Photothermal catalysis is an emerging sub-discipline of heterogeneous catalysis that exploits broad absorption of the solar spectrum to stimulate a combination of thermochemical and photochemical processes, which contribute synergistically to driving catalytic reactions. In particular, it is proving an effective and promising strategy for converting CO2 to synthetic fuels. See full article at Energy & Environmental Science.
December 20th, 2018: The Agnotology of Carbon Dioxide
In 1995, Robert Proctor of Stanford University coined the term, “Agnotology”, refering to the study of how and why we do not know things as a means of addressing the rapid dissemination of misleading, confusing, frightening and false information. Although early works in this field were focused on the understanding the ignorance around smoking and cancer risk, Agnotogy is highly relevant to the current rhetoric surrounding climate change. The reality is that CO2 emissions due to human activity are contributing to climate change and that to limit the warming from pre-industrial levels to 1.5 °C these emissions must be reduced to zero by 2050. Unfortunately, however, denial or acceptance of global warming often stems from selectivity in the search for evidence and political leaning. See full story at: Advanced Science News.
December 18th, 2018: Congratulations to Dr. O’Brien et al. on their paper “Enhanced photothermal reduction of gaseous CO2 over silicon photonic crystal supported ruthenium at ambient temperature”
Solar-driven CO2 hydrogenation can provide a renewable source of fuels and reduce greenhouse gas emissions at industrial scale. The paper investigates the light-driven Sabatier reaction over Ru films sputtered onto silica opal (Ru/SiO2) and inverted silicon opal photonic crystal (Ru/i-Si-o) supports. Under ambient temperature conditions, photomethanation rates over both the Ru/SiO2 and Ru/i-Si-o catalysts were shown to increase significantly with increasing light intensity, and rates as large as 2.8 mmol g−1 h−1 are achieved over the Ru/i-Si-o catalyst. Furthermore, the quantum efficiency of the photomethanation reaction was found to be almost three times larger when measured over the Ru/i-Si-o catalyst as compared to the Ru/SiO2 catalyst. DFT analysis indicate that charged Ru surfaces can destabilize adsorbed CO2 molecules and adsorb and dissociate H
December 6th, 2018: CO2 Conversion and Corrosion: Mind the Gap
The community of scientists and engineers dedicated to the development of synthetic fuels made from CO2 is large and growing. From catalytic synthesis to reactor design, these researchers work on devising strategies to yield the most energy efficient and cost-effective CO2 conversion technologies. There exists, however, another community of experts also dedicated to working on CO2, albeit from a very different perspective: these are the scientists and engineers dedicated to the capture, purification, transportation and distribution of CO2 for either storage or enhanced oil recovery purposes. Their attention is largely focused on the important, and yet often forgotten corrosive nature of CO2 on processing equipment, containers, and pipelines made of carbon steel. Given the current trend, these seemingly disparate fields would greatly benefit by overcoming the CO2 communications gap, which appears to exist between scientists and engineers working on these problems. See full story at: Advanced Science News.
November 19th, 2018: Preventative Care of Our Planet – A Global Renewable Synthetic Fuels Roadmap
Synthetic fuels made using non-fossil renewable sources of energy, could make a significant contribution to achieving the 1.5 °C objective set out by the Paris Climate Agreement. While not widely recognised, technologies for making such synthetic fuels are in an advanced state of technological readiness; however, large-scale production will entail further development of several technologies, decision-making on where to locate different facilities, and the building of infrastructure to transport the fuels to where they are needed. A recent report put forth by Frontier Economics at the commission of the World Energy Council in Germany seeks to develop a dedicated roadmap for establishing a global renewable electricity to fuel industry. See full story at: Advanced Science News.
October 23rd, 2018: U of T Solar Fuels Group Joins Final of the Carbon XPRIZE

The University of Toronto Solar Fuels group has recently joined C4X, an NRG COSIA Carbon XPRIZE finalist, in their effort to create viable carbon dioxide utilization technologies in an effort to alleviate the effects of global warming caused by atmospheric greenhouse gases.
This new partnership brings together several large emitters and technology providers, enabling a clear path forward for validation of new carbon-reducing technologies on an industrial scale. Partners include:
- C4X Technologies Inc., led by Dr. Wayne Song (Toronto, ON)
- PERDC of Ford Motors Canada, led by Dr. Jimi Tjong (Windsor, ON)
- PolyBio Inc., led by Professor Mohini Sain from the Department of Chemical Engineering (Toronto, ON)
- The U of T Solar Fuels group, led by Professor Geoffrey Ozin from the Department of Chemistry (Toronto, ON)
- Walkerville Brewery, led by Neil Bishop
C4X is currently operating in Suzhou, China, and is planning to expand operations to Canada through this partnership.
The University of Toronto Solar Fuels group develops solar-driven technologies for the conversion of greenhouse gases like carbon dioxide into value-added chemicals and products. This opportunity to scale these technologies in a real-world setting is a major step forward in the path to commercialization of this work.
October 20th, 2018: Congratulation to Jinlong Gong for your paper in Angewandte Chemie!
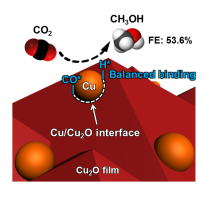
Selectively targeting one high value-added chemical fuel, such as methanol, from CO2 reduction in aqueous solutions remains a grand challenge. By intentionally constructing a well-defined defined Cu/Cu2O interface, the binding strength of surface adsorbed H* and CO* intermediates could be balanced in a photoelectrochemical reduction of CO2 in aqueous solution, leading to methanol production with an impressive Faradaic efficiency of 53.6%. The full paper, entitled “Tuning Cu/Cu2O Interfaces for Reduction of Carbon Dioxide to Methanol in Aqueous Solutions”, can be found at Angewandte Chemie.
October 3rd, 2018: Human Intelligence and Experiential Learning in Materials Discovery
We have arguably reached a point in the evolution of materials chemistry where the discovery of a brand-new material or novel material property is rare. Although there will, of course, always be the occasional eureka moment, current materials research is mainly focused on the design of self-assembled material architectures that yield specific properties to satisfy the function required of an application. An inevitable question in today’s technological context, however, is the role of artificial intelligence in the materials research process. Can AI be trained to accomplish tasks and practice continual learning, intuition, and creativity, in a manner that matches or even supersedes human intelligence? See full story at: Advanced Science News.